A QUALITATIVE STUDY OF PUBLISHED CASE REPORTS OF ADVERSE DRUG REACTIONS
Abstract
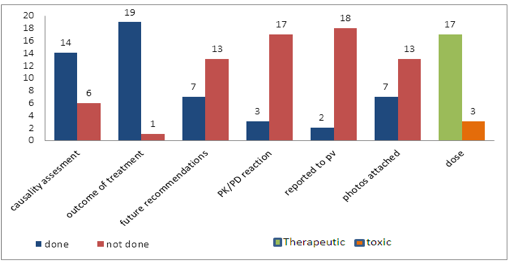
Introduction: To study whether the case reports published in the past and present are meaningful and useful, this study was undertaken. Case reports about adverse drug reactions published in the journals were randomly selected and analyzed using a questionnaire. Methodology: Twenty Case reports reporting the adverse effects to drugs were selected from different journals. They were thoroughly analyzed for their completeness, reason for publication, quality of presentation and ability to highlight the main features to the readers. Salient features mentioned in the case reports were noted. Results: Most of the case reports were the reports of rare ADRs. Most of them adequately described the onset of ADR, dechallenge result and application of causality assessment scales. Areas needing improvement in writing case reports have been identified in this study. Conclusion: Most rare ADRs reported as case reports have to be followed up further to identify any potential signal by pharmacovigilance staff. Case reports can follow a common format which includes the critical aspects like causality assessment, mechanism of ADR, treatment of ADR, dose which causes the ADR, review of relevant literature, details of reporting to nearby Pharmacovigilance centre, PVPI report number, photos wherever applicable, reason for publication and known or new nature of the ADR, and future recommendations by the author of the case report.
Copyright (c) 2019 International Archives of BioMedical and Clinical Research

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





